Chemical reactions and equation
Chemical Reactions
A burning candle is the best example of physical and chemical change. Take a candle and light it. As time passes, we can observe that the candle changes to wax. If you cover the candle with a jar, it will extinguish. In the demonstration, burning of the candle is a chemical change while conversion of the candle to wax is a physical change. In a physical change, there is basically a change of state of the substance but in the case of a chemical change mostly a new substance is formed in which either energy is given off or absorbed. Thus, we can conclude that chemical changes are accompanied by certain physical changes.
Basic Concepts of Chemical Reactions
A Chemical Reaction is a process that occurs when two or more molecules interact to form a new product(s).
Compounds that interact to produce new compounds are called reactants whereas the newly formed compounds are called products.
Chemical reactions play an integral role in different industries, customs and even in our daily life. They are continuously happening in our general surroundings; for example, rusting of iron, pottery, fermentation of wine and so on.
In a chemical reaction, a chemical change must occur which is generally observed with physical changes like precipitation, heat production, colour change etc.
A reaction can take place between two atoms or ions or molecules, and they form a new bond and no atom is destroyed or created but a new product is formed from reactants.
The rate of reaction depends on and is affected by factors like pressure, temperature, the concentration of reactants.
Chemical Equations
Due to the vast amounts of chemical reactions happening around us, a nomenclature was developed to simplify how we express a chemical reaction in the form of a chemical equation. A chemical equation is nothing but a mathematical statement which symbolizes the product formation from reactants while stating certain condition for which how the reaction has been conducted.
The reactants are on the left-hand side whereas the products formed are on the right-hand side. The reactants and products are connected by a one-headed or two-headed arrows. For example, a reaction
A + B → C + D
Here, A and B are the reactants, which react to form the products C and D. In an actual chemical equation, reactants are denoted by their chemical formula. In order to assure the law of conservation of mass, a chemical equation must be balanced i.e. the number of atoms on both sides must be equal. This is the balancing of the equation. Let us consider an actual chemical reaction between Methane(CH₄) and Oxygen (O2)
Types of Chemical Reactions
The basis for different types of reactions is the product formed, the changes that occur, the reactants involved and so on. Different types of reactions are
Combustion reaction
Decomposition reaction
Neutralization reaction
Redox Reaction
Precipitation or Double-Displacement Reaction
Synthesis reaction
Balancing Chemical Equations
Balancing chemical equations involves the addition of stoichiometric coefficients to the reactants and products. This is important because a chemical equation must obey the law of conservation of mass and the law of constant proportions, i.e. the same number of atoms of each element must exist on the reactant side and the product side of the equation. Two quick and easy methods of balancing a chemical equation are discussed in this article. The first method is the traditional balancing method and the second one is the algebraic balancing method.
Related Terminology
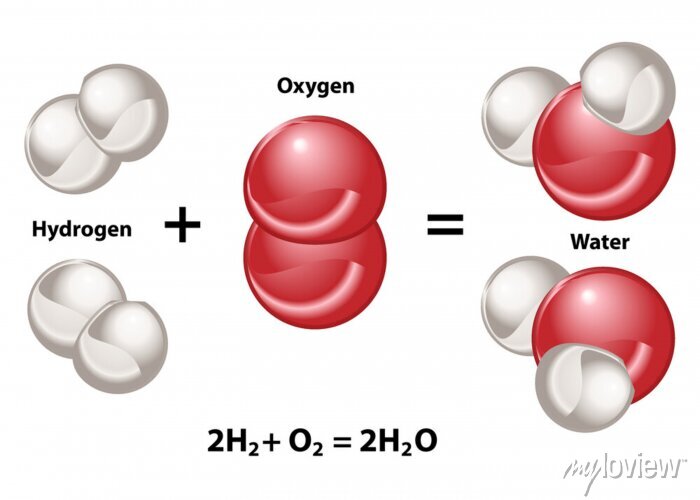
Chemical Equation A chemical equation is a symbolic representation of a chemical reaction in which the reactants and products are denoted by their respective chemical formulae. An example of a chemical equation is 2H2 + O2 → 2H2O which describes the reaction between hydrogen and oxygen to form water The reactant side is the part of the chemical equation to the left of the ‘→’ symbol whereas the product side is the part to the right of the arrow symbol.
Stoichiometric Coefficient
A stoichiometric coefficient describes the total number of molecules of a chemical species that participate in a chemical reaction. It provides a ratio between the reacting species and the products formed in the reaction. In the reaction described by the equation CH4 + 2O2 → CO2 + 2H2O, the stoichiometric coefficient of O2 and H2O is 2 whereas that of CH4 and CO2 is 1. The total number of atoms of an element present in a species (in a balanced chemical equation) is equal to the product of the stoichiometric coefficient and the number of atoms of the element in one molecule of the species. For example, the total number of oxygen atoms in the reacting species ‘2O2’ is 4. While balancing chemical equations, stoichiometric coefficients are assigned in a manner that balances the total number of atoms of an element on the reactant and product side.