Physical and Chemical Changes

Difference Between Physical and Chemical Change
There are many differences between physical and chemical changes and it is important to understand them to be able to understand these concepts clearly. The comparisons and differences between physical and chemical changes are given below along with their examples. To understand physical and chemical properties and changes better, it is important to know what they are. Visit physical and chemical changes to know more about them in detail. In this article, the differences between physical changes and chemical changes are provided in a tabular format.
Table of Contents Differences Between Physical and Chemical Change
Recommended Videos
Frequently Asked Questions – FAQs
Differences Between Physical and Chemical Change
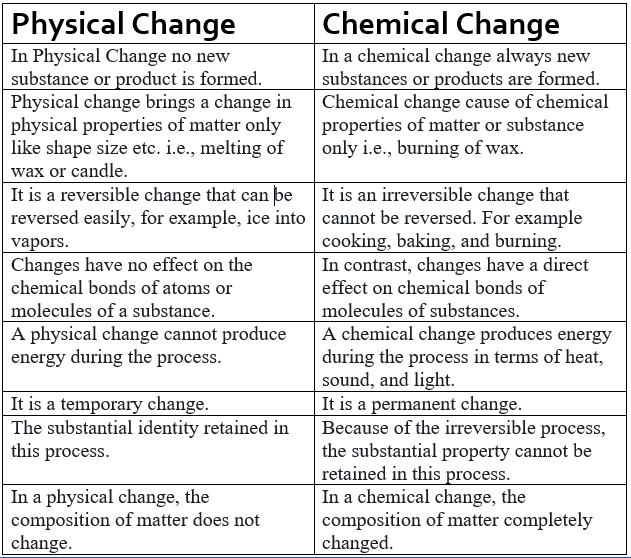
Thus, it can be understood that the primary difference between physical and chemical changes is that physical changes are reversible, whereas chemical changes are usually not.
Frequently Asked Questions – FAQs
Q1 How can you tell the difference between physical and chemical changes?
The appearance or form of matter changes during a physical change, but the type of matter in the substance does not. A chemical change, on the other contrary, results in the creation of at least one new substance with new properties.
Q2 Why is it important to know the difference between physical and chemical changes?
It’s essential to recognise the difference between chemical and physical changes. Several changes are obvious, but there are some fundamental concepts to be aware of. Physical changes usually refer to changes in the physical state of stuff. When two or more molecules interact, chemical changes occur on a molecular level.
Q3 What are the examples of physical and chemical changes?
Examples of chemical changes would be burning, cooking, rusting, and rotting. Examples of physical changes could be boiling, melting, freezing, and shredding. Most physical changes can be reversed if sufficient energy is provided.
Q4 How to tell whether it’s a physical or chemical change?
Check for indications that a chemical change has taken place. The following are indications of a chemical change:
•Gas is created. Bubbles can occur in liquids.
•An odour is created.
•The colour of the substance changes.
•Sound is generated.
•There is a shift in the temperature. The environment either heats up or cools down.
•Light is generated.
•A precipitate develops.
•Reversing the change is difficult or impossible.
Q5 What are three forces that can cause a physical change?
Forces such as motion, temperature, and pressure can create physical changes. Oxygen in the air reacts with sugar, and the chemical bonds are destroyed.