Periodic classification of elements
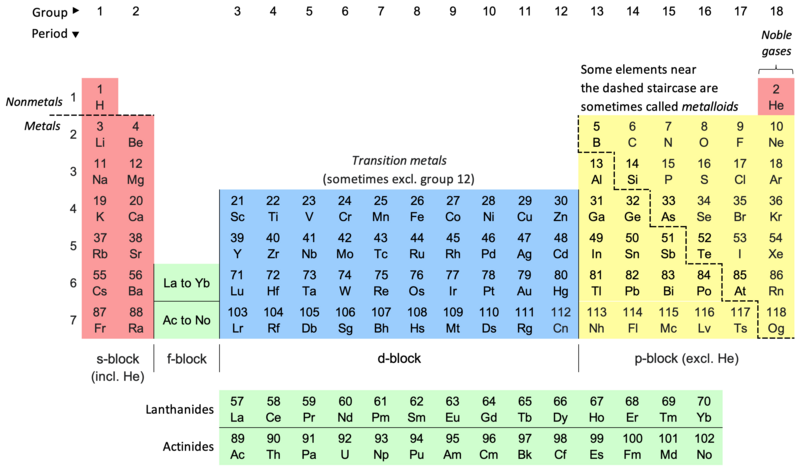
Periodic classification of elements
All existing matter in our surroundings is made up of basic units known as elements. Initially, in 1800, only 31 chemical elements were discovered. After some advancement in technology in 1865, about 63 more elements were discovered. This created the need for the periodic classification of elements. Presently, there are 118 elements known to us. Out of these 118 chemical elements, some elements are man-made.
When there were only 31 elements it was relatively easy to study the properties of these chemical elements individually. Now the number of elements has raised to 118, it would be very cumbersome to study the properties of every element individual. In order to ease the work, scientists started thinking about a method so that the study of elements can be simplified. They decided to organize the elements in a periodic table according to the information available about the elements and various characteristics shown by them. It was observed that elements show periodicity in their properties. Many tables were made to arrange the elements in an ordered manner based on their characteristics to study the properties of elements in a fixed pattern.
Periodic Classification of Elements Characteristics
In the long form periodic table the elements are arranged in the order of their atomic numbers. Atomic number of an element is equal to the number of protons inside the nucleus of its atom.
The general features of the long form periodic table are:
There are in all, 18 vertical columns and 18 groups in the long form periodic table.
These groups are numbered from 1 to 18 starting from the left.
There are seven horizontal rows called periods in the long form periodic table. Thus, there are seven periods in the long form periodic table.
The elements of Groups 1, 2 and 13 to 17 are called the main group elements. These are also called typical or representative or normal elements.
The elements of Groups 3 to 12 are called transition elements.
Elements with atomic number 58 to 71 (Ce to Lu) occurring after lanthanum (La) are called lanthanides. Elements with atomic numbers 90 to 103 (Th to Lw) are called actinides. These elements are called f-block elements and also as inner transition elements.
Today we study the elements with the help of the modern periodic table. Periodic classification of elements is the method by which elements are grouped on the basis of their characteristics i.e. we keep the elements that are alike in one group and the rest of the elements in the other group. Some empty spaces have been left in the periodic table so that the elements that will be discovered in the future can be placed without disturbing the trending periodicity of the elements.
Significance of the Periodic Classification of Elements
Makes the study of elements easy: Classification of elements in groups provide us with a fixed pattern in which the elements change their properties periodically. The periodic table made the study of the physical and chemical properties of elements simple and organized. We can now just go to the group and see the properties of the elements of the periodic table or predict the properties of an element if we know the characteristics of other elements present in the same group.
Helps in discovering new elements: Although there are so many elements that are already discovered, there are chances that new elements can be discovered. Scientists can take the help of a periodic table and know the trending characteristics based on the properties of elements and hence can identify the new elements with the existing ones. Besides, researchers are continuously striving to discover new elements and explore their properties.
Newland’s Law of Octaves and Dobereiner’s Triads
What are Dobereiner’s Triads?
Dobereiner’s triads were groups of elements with similar properties that were identified by the German chemist Johann Wolfgang Dobereiner. He observed that groups of three elements (triads) could be formed in which all the elements shared similar physical and chemical properties. Dobereiner stated in his law of triads that the arithmetic mean of the atomic masses of the first and third element in a triad would be approximately equal to the atomic mass of the second element in that triad. He also suggested that this law could be extended for other quantifiable properties of elements, such as density. The first of Dobereiner’s triads was identified in the year 1817 and was constituted by the alkaline earth metals calcium, strontium and barium. Three more triads were identified by the year 1829. These triads are tabulated below.
Newland’s Law of Octaves In the year 1864, the British chemist John Newlands attempted the 62 elements known at that time. He arranged them in an ascending order based on their atomic masses and observed that every 8th element had similar properties. On the basis of this observation, Newland’s law of octaves was formulated. The law of octaves states that every eighth element has similar properties when the elements are arranged in the increasing order of their atomic masses. An illustration detailing the elements holding similar properties as per Newland’s law of octaves is provided below.
Newland's Law of Octaves
Newlands compared the similarity between the elements to the octaves of music, where every eighth note is comparable to the first. This was the first attempt at assigning an atomic number to each element. However, this method of classifying elements was met with a lot of resistance in the scientific community.
Limitations of Newland’s Law of Octaves
The key shortcomings of Newland’s law of octaves are listed below.
Several elements were fit into the same slots in Newland’s periodic classification. For example, cobalt and nickel were placed in the same slot.
Elements with dissimilar properties were grouped together. For example, the halogens were grouped with some metals such as cobalt, nickel and platinum.
Newland’s law of octaves held true only for elements up to calcium. Elements with greater atomic masses could not be accommodated into octaves.
The elements that were discovered later could not be fit into the octave pattern. Therefore, this method of classifying elements did not leave any room for the discovery of new elements.