Mixtures
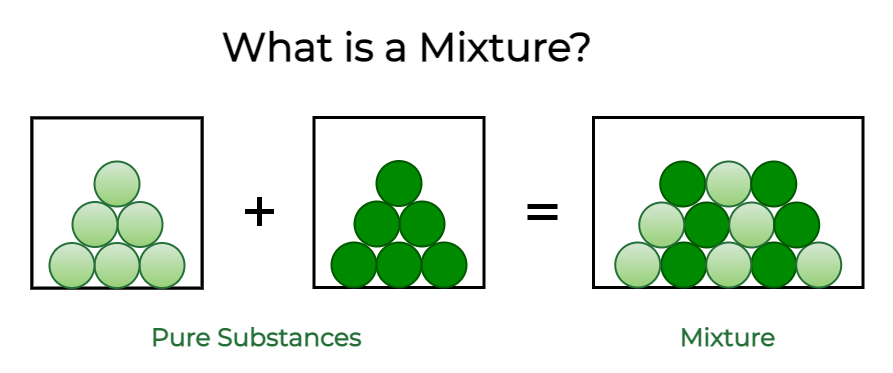
What is Mixtures?
In chemistry, when two or more substances mix with each other without participating in a chemical change, the resulting substance is called a Mixture.
The result formed due to the combination of substances does not lose its individuality nor are they combined chemically. Mixtures are the one product of a mechanical blending or mixing of chemical substances such as elements and compounds.
General Properties of Mixtures
Mixtures are made up of two or more substances that are not chemically combined with each other. The properties of mixtures are listed below.
• The components of a mixture each keep their original properties.
• The separation of components can be easily done.
• The proportion of the components is variable.
Examples of Mixtures
Crude oil: A mixture of organic compounds (mainly hydrocarbons)
Seawater: A mixture of various salt and water.
Air: a mixture of various gases like oxygen, carbon dioxide, nitrogen, argon, neon, etc.
Ink: A mixture of coloured dyes.
Gunpowder: A mixture of sulfur, potassium nitrate and carbon.
There are two main types of mixtures: homogeneous mixtures and heterogeneous mixtures. The types of mixtures are discussed below.
1. What is a Heterogeneous Mixture?
A mixture of sand mixed with salt is an example of a heterogeneous mixture. Heterogeneous mixtures possess different properties and compositions in various parts i.e. the properties are not uniform throughout the mixture.
Examples of Heterogeneous mixtures – air, oil, water, etc.
2. What is a Homogeneous Mixture?
Sugar mixed with water is the most common example of a homogeneous mixture. Homogeneous mixtures can be defined as the mixtures which possess the same properties and combination throughout their mass.
Examples of Homogeneous mixtures – alloys, salt, and water, alcohol in water, etc.
Characteristics of Mixtures
The constituents of a mixture are not present in a fixed ratio. The various characteristics of mixtures are discussed below.
• There is no chemical force acting between the two or more substances that are mixed, but they still exist together.
• They can either be heterogeneous or homogeneous in nature.
• The proportions of the substances vary in an indefinite manner.
• The properties of the mixture depend upon the individual components.
• The constituents of the mixture can be separated by physical methods.
• The boiling point and the melting point of the mixture depends upon the characteristic of the constituents.
• During the formation of a mixture, there is no change in energy.
• All the states of matter (solid, liquid, gases) can combine to form mixtures.
It can be concluded that almost everything in our vicinity is nothing but a mixture. For example, the food we eat is a mixture of ingredients, the atmospheric air we breathe is a combination of gases and the fuel we use in locomotives is a heterogeneous mixture.
Frequently Asked Questions – FAQs
Q1
What are the Categories that Mixtures can be Classified Into?
Q1 What are the Categories that Mixtures can be Classified Into?
Mixtures can be classified into the following categories: Homogeneous mixtures – possess the same properties and combination throughout their mass. Heterogeneous mixtures – possess different properties and compositions in various parts.
Q2 What are the Key Properties of Mixtures?
Each individual component of a mixture retains its original physical and chemical properties. Also, it is generally easy to separate the individual components of a mixture. Finally, the proportion of the components throughout the mixture varies.
Q3 Which is an example of a mixture?
Combining two or more substances shapes a mixture. Regardless of where you test it, a homogeneous solution appears uniform. Homogeneous mixtures are sources of soil, saline solution, most alloys and bitumen. Sand, oil and water and chicken noodle soup are examples of heterogeneous mixtures.
Q4 Is Vinegar a mixture?
Vinegar is a homogeneous mixture of water and acetic acid. It is a solution because the mixture created has only one phase. Through combining two or more chemical substances, mixtures are formed. If the material has more than one phase, it is otherwise referred to as a mixture.
Q5 What is a simple mixture?
A mixture is a material composed of two or more simpler substances in chemistry. Such materials can be compounds or chemical elements. A mixture of liquids, solids, or gases can be produced. When sugar is put in water, for example, it forms a mixture, then it dissolves to create a solution.